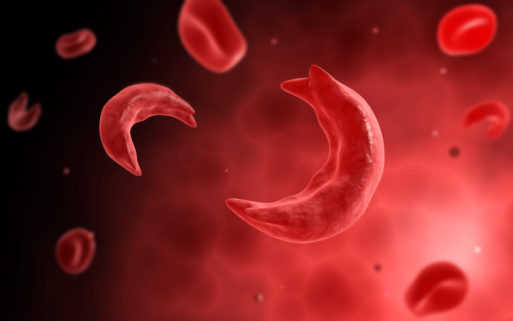
Sickle-shaped red blood cells under a microscope
Credit: africanleadership.co.uk
A new drug, Endari, has just received FDA approval for the treatment of sickle cell disease. The medication is the first new sickle cell therapy to be approved by the Food and Drug Administration in over 20 years.
Endari is manufactured by a privately held biotech company, Emmaus Medical, Inc. of Torrance, California. Emmaus has marketed a nutraceutical-grade version of the drug, L-glutamine, (NutreStore®) as a treatment for short bowel syndrome since 2004. L-glutamine is also widely available without a prescription at drugstores across the United States, albeit in much smaller doses than those the FDA recommends for sickle cell disease.
The FDA granted Endari Orphan Drug status, a designation reserved for medicines that treat serious diseases that affect only a small portion of the population. Sickle cell disease affects about 100,000 men, women and children in the United States, most of them of African American descent.
About Sickle Cell Disease
Sickle cell disease, or sickle cell anemia, is an inherited disorder of hemoglobin, a component of red blood cells. In persons with the disease, abnormal hemoglobin causes the red blood cells to become fragile and misshapen, especially when the body is under stress (such as from dehydration or an infection). These fragile cells break easily, which causes anemia (a decrease in the number of circulating red blood cells). And because of their abnormal “sickle” shape, they easily become lodged in small blood vessels throughout the body, causing severe pain. The disease also causes organ dysfunction, especially in the liver, spleen and lungs.

Endari is the first sickle cell treatment approved for children
Credit: ladybud.com
People with sickle cell disease spend a great deal of time in the hospital and generally suffer a diminished quality of life. Fatigue and pain are common, and life-threatening complications, such as severe anemia, heart failure, lung injury and bacterial pneumonia often occur. Although life expectancy for sickle cell patients has improved considerably over the last few decades, their average lifespan is still only about 50 years.
Sickle cell disease is caused by an autosomal recessive gene, which means a person must inherit an abnormal gene from both parents to have the disease. People who have one copy of the abnormal gene do not have symptoms of sickle cell disease.
What Endari Does
Endari is not a cure for sickle cell disease. However, in clinical trials, it decreased the number of painful “crises” by about one per year in people who had at least two attacks in the previous 12 months. The FDA felt this was a significant benefit, especially in patients who had four or more painful crises per year.
People treated with Endari also had fewer hospital visits for pain treatment; fewer hospitalizations for sickle cell pain; and spent fewer days in the hospital than those treated with a placebo. Perhaps most significantly, they also had fewer episodes of acute chest syndrome, the leading cause of death in adults with sickle cell disease.
Endari is not yet commercially available. Emmaus plans to begin marketing the drug in the fourth quarter of 2017. The company has not yet announced the price, but Endpoints reports that the company anticipates “the list price range will be approximately $11,000 to $18,000 per year depending on dosing.” Insofar as most people with sickle cell disease are on Medicare or Medicaid, that price point may be a hard sell.

 FDA Approves New Drug for Sickle Cell Disease
FDA Approves New Drug for Sickle Cell Disease



 The Spiritual Symbolism of Cardinals
The Spiritual Symbolism of Cardinals
 Meaning-Focused Grief Therapy: Imaginal Dialogues with the Deceased
Meaning-Focused Grief Therapy: Imaginal Dialogues with the Deceased














