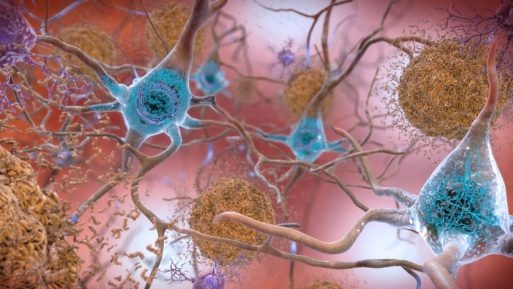
Abnormal proteins, including beta amyloid (brown) and tau (blue) appear in the brain in a person with Alzheimer’s disease
For decades, scientists have been researching potential treatments for Alzheimer’s disease, many of which have targeted a substance known as beta-amyloid. A sticky protein that accumulates in the brains of people with AD, beta amyloid has been shown to clump together in oddly formed configurations known as amyloid plaques both inside and outside nerve cells in the brain. Because these plaques are consistently seen in people with Alzheimer’s symptoms, they have long been believed to be a causative agent in Alzheimer’s development. As a result, beta-amyloid has been the target of nearly all pharmaceutical research aimed at developing treatments for Alzheimer’s disease — now the sixth leading cause of death in the world.
Sadly, after countless failed clinical trials and billions of dollars in federal funding, the pharmaceutical industry has failed to come up with even one drug that slows the progression of the deadly brain disease. In fact, the latest of only six drugs approved by the FDA for Alzheimers treatment, Biogen’s Aduhelm, has been the subject of an ongoing scandal since it was granted approval in 2021. More recently, the research behind another widely touted Alzheimer’s treatment, Cassava Sciences’ drug Simufilam, has been called into question. The U.S. Justice Department is currently investigating Cassava Sciences for possible criminal wrongdoing in connection with its claims about the drug.
Perhaps even more disturbingly, recent evidence uncovered by a 6-month investigation by Science magazine indicates that the results of a 2006 study that has informed decades of Alzheimer’s research may have been faked.

Sylvain Lesne, Ph.D.’s Alzheimer’s research is now in question
Credit: University of Minnesota Medical School
At the heart of that scandal is a study led by Sylvain Lesne, Ph.D., a professor of neuroscience at the University of Minnesota Medical School, that was published in the prestigious journal Nature in 2006. Lesne and his colleagues identified a specific variant of the beta-amyloid molecule, termed Aβ*56, that was responsible for memory loss in transgenic mice “independently of plaques or neuronal loss.” The paper was accompanied by images showing the nature of the protein accumulation that Lesne et.al. identified. Now, according to Science and several other experts in the field of Alzheimer’s research, it appears that many of those images were doctored to support Lesne’s claims.
Lesne’s findings were never duplicated — another red flag that the research was, at best, flawed. Nevertheless, many in the scientific community, which even 16 years ago was desperate for a target for Alzheimer’s treatment, seized on the idea. His paper has been cited over 2,000 times in various scientific publications, even as some of his colleagues wrote it off as meaningless after they failed to replicate his results.
The Amyloid Hypothesis
While the discovery of Lesne’s allegedly manipulated images is extremely disturbing, it is not the “deal breaker” that some might suspect. Many other respected Alzheimer’s researchers have performed rigorous studies that support what is known as the “amyloid hypothesis,” a theory that identifies beta-amyloid as a contributing factor in a cascade of changes that result in the symptoms associated with AD. What is controversial, however, is the importance of amyloid in the development of the disease. As many studies have confirmed, the mere presence of amyloid plaques in the brain does not correlate reliably with the presence of dementia, nor does the amount of plaque deposition in an individual with Alzheimer’s disease correlate reliably with the degree of cognitive impairment they experience.
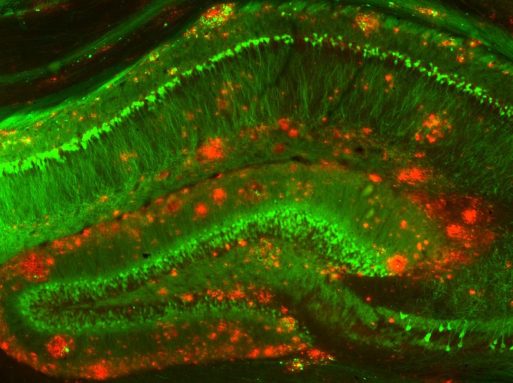
A mouse model of Alzheimer’s disease was used in the experiments that showed beta-amyloid’s toxic effects
Credit: NIH Image Gallery via Flickr
And, of course, the failure of any drug that has targeted beta-amyloid to consistently reduce Alzheimer’’s symptoms in humans speaks volumes on its own.
Undermining the Public Trust
As the scientific community weighs the importance of these ongoing scandals, what is perhaps most important is the fact that the level of dishonesty they reveal inevitably undermines the public trust. We live in an age in which many Americans have lost faith in once-revered institutions, and scientific research is often viewed with skepticism or even disdain. Knowledge, by nature, evolves, and what appeared true yesterday can be disproved today. But deception at any stage of the scientific process is impossible to excuse. As reputable scientists search for treatments for Alzheimer’s disease, cancer and other devastating conditions, they must ensure that they and their colleagues uphold the highest standards of conduct and supply truthful, accurate information about what they know and what they don’t know. Anything less is simply unacceptable. and ultimately harmful to us all.

 Scandals Around Alzheimer’s Research Rock the Scientific Community
Scandals Around Alzheimer’s Research Rock the Scientific Community


 Who Cares for the Caregivers?
Who Cares for the Caregivers?
 Final Messages of the Dying
Final Messages of the Dying















