Dementia causes many troublesome and difficult to manage symptoms. In addition to memory loss, confusion and difficulty concentrating, many dementia patients exhibit challenging behaviors, such as depression, irritability, agitation and wandering. In some patients, these are accompanied by symptoms of psychosis, such as visual hallucinations, delusions and paranoia.
 Known collectively as behavioral and psychotic symptoms of dementia, these psychiatric symptoms are present to some degree in about 90 percent of persons with moderate-to-advanced dementia, the Alzheimer Association reports. Psychotic symptoms are particularly common in people with Parkinson’s disease dementia, a specific type of dementia characterized by abnormal deposits of the protein alpha-synuclein, known as Lewy bodies, in the brain.
Known collectively as behavioral and psychotic symptoms of dementia, these psychiatric symptoms are present to some degree in about 90 percent of persons with moderate-to-advanced dementia, the Alzheimer Association reports. Psychotic symptoms are particularly common in people with Parkinson’s disease dementia, a specific type of dementia characterized by abnormal deposits of the protein alpha-synuclein, known as Lewy bodies, in the brain.
Sadly, these challenging behaviors are often very difficult to treat. At one time, doctors routinely prescribed antipsychotic medications to calm the patient and provide a measure of respite to caregivers. More recently, however, research has shown a higher risk of mortality in elderly dementia patients treated with antipsychotic drugs. Thus, the practice of routinely drugging difficult dementia patients with antipsychotics has tapered off.
But apparently not enough.
New Drug – More Deaths
In June 2016, a new medicine designed to treat psychosis in dementia patients with Parkinson’s disease hit pharmacy shelves. The drug, pimavanserin (Nuplazid) received FDA approval in early 2016. But unlike most new drugs, which undergo rigorous and lengthy clinical trials, Nuplazid received approval based mainly on a single 6-week study involving about 200 dementia patients performed by the drug’s manufacturer, Acadia Pharmaceuticals. The “fast track” approval came after the drug was granted “breakthrough status” by the FDA in 2012.
Many Reservations
Not everyone at the FDA was enthusiastic about Nuplazid, according to an April 2018 report by CNN. Although the vote to approve was 12-2, several members of the review panel had serious reservations about the drug. Dr. Paul Andreason, for example, warned that patients taking Nuplazid during the company’s clinical trials experienced serious adverse events, including death, at more than double the rate of those taking a placebo. Further, he said, previous testing by the company had shown the drug provided no benefit to patients at all. He was not convinced, based on the results of one small study, that the benefits of the drug outweighed the risks.
The elderly often take multiple medications, all carrying different risks
Even panelists who voted to approve Nuplazid voiced concerns.”I guess I’m hoping that the risks are going to be small, and I think the benefits for some of these people who are very sick and whose families are affected by this, I think they’re probably willing to take that risk,” one physician said. Another frankly asked the FDA to “consider a large observational study so we can ensure that, once it goes into real-world use, that the benefits will outweigh the risks.”
But after listening to desperate family members begging for access to the new drug, the committee relented, and Nuplazid was approved.
Hundreds of Deaths
Fast forward to 2018, and hundreds of reports of adverse events in patients taking Nuplazid have been submitted to the FDA. These include reports of relatively minor side effects such as nausea and vomiting, as well as life-threatening incidents and actual deaths. An analysis of the FDA data by the independent watchdog group the Institute for Safe Medical Practice warned in March 2017 that 244 deaths in dementia patients taking Nuplazid had already occurred. Additionally, there were over 1,000 reports that the drug caused worsening hallucinations, increased confusion, or simply didn’t work.
Since that analysis was released, the number of dementia patients who have died while taking Nuplazid has risen to over 700, according to CNN. Nuplazid was the only drug listed as “suspect” in over 500 of those deaths.
No FDA Action
To be clear, adverse event reports submitted to the FDA are not in themselves proof that a drug caused the event. Elderly dementia patients typically have many co-existing health conditions and are often taking multiple drugs. Further, psychosis in people with Parkinson’s disease usually occurs when the disease is quite advanced, when patients are already at higher risk of death.

FDA head Scott Gottleib
The issue is further complicated by the fact that adverse event reports are rarely investigated by the FDA. So the details surrounding patient deaths and other negative outcomes are not at all clear. Thus, the FDA has taken no further action, other than to state that “at this time” the agency has “not identified a specific safety issue that is not already adequately described in the product labeling.” In fact, Acadia is proceeding with clinical trials in patients with other forms of dementia, including Alzheimer’s disease. According to the company, in two trials involving about 300 patients, the number of patient deaths did not differ significantly between those taking Nuplazid and those taking a placebo. Based on this data, the FDA recently gave Nuplazid “breakthrough status” again, meaning it will probably be approved for even wider use.
Still, independent consumer health advocates and experts interviewed by CNN believe that Nuplazid needs more study, and that patients lives are at risk. Diana Zuckerman, the founder and president of the nonprofit National Center for Health Research, said in a statement,” This is almost unheard of, to have this many deaths reported….You just don’t see this with most new drugs — you don’t see all these reports — so you have to take it seriously.”
These sentiments were echoed by geriatric psychiatrist and and former FDA medical officer Susan Molchan, who told CNN she questioned the drug’s safety and wondered if patients and their families were aware of the risks associated with taking the drug.
“This is exactly what I thought was going to happen,” added Paul Andreason, the FDA doctor who joined one other colleague in voting against approval of the drug.
Not an Isolated Instance
Perhaps the most alarming aspect of the Nuplazid scenario is that it is not an isolated instance of lax FDA oversight of a drug that targets elderly dementia patients who cannot speak for themselves. In January, SevenPonds reported on the rampant use of the drug Neudexta, a medication that initially received FDA approval for use in patients with a very specific condition known as pseudobulbar affect, which primarily impacts people with multiple sclerosis and ALS. The drug was tested on the elderly only once, in a 10-week trial involving 300 patients. Yet, the FDA recently expanded its approval so the drug may be given to patients with a variety of neurological conditions, including dementia and Alzheimer’s disease. Since then, Neudexta has become a staple in nursing homes, where staff routinely use it to calm patients who are agitated or upset. And not surprisingly, thousands of adverse event reports have been submitted to the FDA, which has taken no action to date.
Drugs Replacing Staff
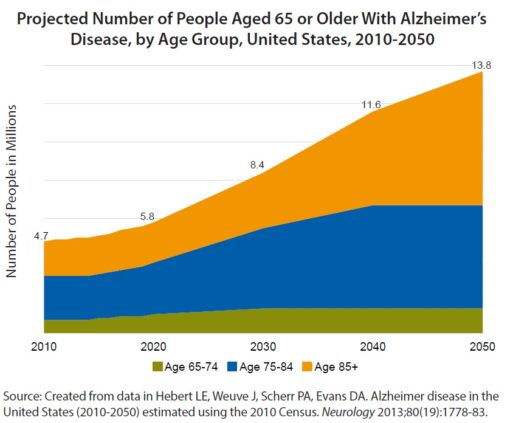
According to the Alzheimer’s Association, 82 percent of persons with dementia are over 75 years old. Thus, as America ages over the coming decades, so too will to the number of elderly persons with dementia needing care. Not all of these people will suffer from psychotic symptoms such as hallucinations and delusions. But most will exhibit troublesome behaviors, such as poor impulse control, aggression and agitation at times. And if current trends continue, more and more of them will be drugged into a stupor with medicines that may very well increase their risk of negative outcomes and even death.
This country can do better. But not until we start putting people above profits and demand that our elderly receive the compassionate care they need.

 Are Dementia Patients Being Drugged to Death?
Are Dementia Patients Being Drugged to Death?


 Final Messages of the Dying
Final Messages of the Dying
 Will I Die in Pain?
Will I Die in Pain?















