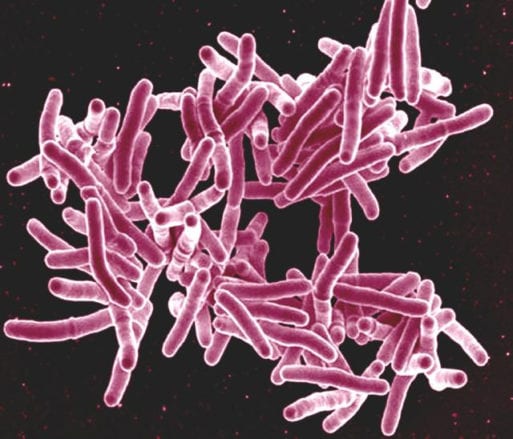
Mycobacterium tuberculosis, the organism responsible for XDR TB, viewed under a microscope
Credit: CDC.gov
Researchers in South Africa have reported the development of a new drug regimen that cures the deadly infection known as extensively drug-resistant tuberculosis or XDR TB. The three-drug combination is said to be 90 percent effective against a disease that previously had a cure rate of about 20 percent.
Even more remarkably, the treatment regimen consists of three drugs, or five pills a day, taken for a period of six months. Before the study, patients with XDR TB suffered through years of a 40-pill-a-day drug regimen and injections that left them so debilitated most of them simply stopped treatment and waited to die.
A Worldwide Epidemic
Once the leading cause of death across the globe, tuberculosis has been largely controlled in most of the developed world since the discovery of antibiotics that cured the disease. According to the U.S. Centers for Disease Control and Prevention, the U.S. TB rate was 2.8 cases per 100,000 in 2016, one of the lowest in the world.
But while the United State has managed to successfully combat the illness once dubbed the “White Plague,” much of the globe hasn’t fared as well. In 2014, TB became the most deadly infectious disease on the planet, killing 1.5 million people worldwide — 3 million more than HIV/AIDs. More alarmingly, the last decade has seen the emergence of a new strain of tuberculosis that is resistant to every antibiotic used to combat the disease, XDR TB. The illness has a history of killing nearly everyone who develops it, usually within one month. Even with aggressive treatment, only about 30 percent survive.
A Promising Trial
Dubbed NixTB, the promising XDR TB study is being led by Dr. Pauline Howell, a tuberculosis researcher at Sizwe Tropical Diseases Hospital in Johannesburg, South Africa. There, XDR TB patients are housed in a separate unit for a minimum of four months while being treated for their disease. (They can’t be allowed home because the risk of spreading the illness is too great.) Each patient has a private room with a bathroom, a large window with a view of the lawn, and a door that leads outside. Those who are strong enough can work in the garden, take classes or play foosball to pass the time.

Patients with XDR TB are treated at the Sizwe Tropical Diseases Hospital in Johannesburg, South Africa
Credit: egyptindependent.com
Patients at Sizwe take three drugs: bedaquiline, pretomanid and linezolid (abbreviated as BPaL). The combo differs from most XDR TB regimens in that all three drugs kill tuberculosis bacteria, whereas the standard treatment consists of two strong drugs that destroy the bacteria and one that simply keeps it from multiplying.
It’s a “bold” regimen, Dr. Howell told the New York Times “because it’s three killer drugs instead of two killers plus some supportive ones.”
Questions About Safety
Still, the new treatment is not without its dangers. Long-term administration of linezolid has been shown to destroy the nerves in the feet of some patients, making it difficult for them to walk. It can also suppress the bone marrow, which may lead to a shortage of infection-fighting white blood cells and platelets, which help the blood to clot. Researchers recently started a second trial, ZeNix, to determine the safest dose of the drug.
More concerning, according to some experts, is the inclusion of pretomanid, which has not been carefully investigated by the FDA. Developed by the TB Alliance, a nonprofit based in New York, pretomanid is a novel antibacterial agent in the nitroimidazole class, which includes the widely prescribed antibacterial/antifungal agent metronidazole (Flagyl).
“Pretomanid looks like a promising drug, but it’s being rushed forward,” said Lindsay McKenna, co-director of the tuberculosis project at the Treatment Action Group in a statement to the NY Times. “… We don’t want to see the FDA lower the bar for approval,” she said in July. McKenna called for more testing before the drug was approved.
Dr. Mel Spigelman, current president and CEO of the TB Alliance, strongly disagreed. Emphasizing that a full clinical trial would cost $30 million and take another five years to complete, he said, “Put yourself in a patient’s position. Offered a choice between three drugs with a 90 percent cure rate, and 20 or more with less chance of cure — who would consent to randomization?”

Tsholofelo Msimango talks about the ordeal of living with XDR TB
Credit: Aleksandra Sagan/The Canadian Press
The FDA sided with Dr. Spigelman, voting on Aug. 14, 2019, to approve BPaL under the Limited Population Pathway for Antibacterial and Antifungal Drugs (LPAD pathway) established under the 21st Century Cures Act. The LPAD “encourages further development of antibacterial and antifungal drugs to treat serious, life-threatening infections that affect a limited population of patients with unmet needs,” according to the FDA. The World Health Organization is expected to quickly follow the FDA’s lead and approve the drug regimen for patients with drug-resistant TB.
For patients like Tsholofelo Msimango, who was one of 109 patients treated during the initial Nix trial, the decision is welcome news. Just 57 pounds when she was diagnosed with XDR TB five years ago, she went to Sizwe expecting never to return “They told my parents to fix the insurance because I would die,’ she told the Times.
Today, she is 25 years old and has a young son. At 103-pounds, she is healthy and tuberculosis free.

 Science Strikes a Blow Against Deadly Strain of TB
Science Strikes a Blow Against Deadly Strain of TB


 Final Messages of the Dying
Final Messages of the Dying
 Will I Die in Pain?
Will I Die in Pain?















