Credit: upi.com
A new study conducted by Columbia University Medical Center Researchers and published this week in the journal Cell Systems identifies a gene that seemingly dictates how fast a brain ages. The gene, called TMEM106B, begins to effect the brain once a person reaches the age of about 65, and may modify the risk of neurodegenerative diseases.
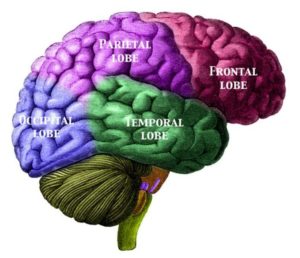
Variants of TMEM106B affect aging in the frontal cortex, the region of the brain responsible for higher mental processes such as language, memory and decision making. Researchers found that people who have two ‘bad’ copies of TMEM106B have a frontal cortex that looks 10 to 12 years older than people of the same age who have two working copies of the gene.
“If you look at a group of seniors, some will look older than their peers and some will look younger,” said Dr. Asa Abeliovich, co-author of the study and professor of pathology and neurology at the Taub Insistitue for Alzheimer’s Disease and the Aging Brain at CUMC. “The same differences in aging can be seen in the frontal cortex.” Until the age of 65, says Dr. Abeliovich, “everybody’s in the same boat, and then there’s some yet-to-be-defined stress that kicks in. If you have two good copies of the gene, you respond well to that stress. If you have two bad copies, your brain ages quickly.”

Credit: dana.org
To carry out the study, Drs. Abeliovich and Herve Rhinn, PhD, analyzed genetic data from autopsied human brain samples collected from 1,904 different people not affected by neurodegenerative diseases. The researches examined the transcriptomes (initial products of gene expression) taken from their samples. This created a composite picture of the average brain biology of people at a given age. The researchers then compared each person’s transcriptome with the average transcriptome of people at the same age. The researchers focused on about 100 genes whose expression increases or decreases with aging.
Different Rates of Brain Aging Seen
From this information, scientists formulated a measure they called “differential aging” — the difference between a person’s apparent (biological) brain age and their chronological brain age. TMEM106B stood out as a genetic driver of differential aging. It seemingly controls the brain’s levels of inflammation and neuronal loss. One form of the gene is associated with increased brain aging. The other form is protective, preventing the acceleration of aging in the brain.
According to study co-author Dr. Rhinn, who is an assistant professor of pathology and cell biology at the Taub Institute, the TMEM106B genetic variant is very common. “About one-third of people have two copies and another third have one copy,” said Dr. Rhinn. “From what we could see, the effect of the [TMEM106B] risk allele is additive, in the sense that the brain of elderly people with two copies of the risk allele ‘looks’ five years older than the [brains] of people with only one copy of risk allele. And [they] themselves ‘look’ five years older than people with no risk allele.”
As a result of the study’s findings, TMEM106B has the potential to be a new biomarker for assessing the need for anti-aging interventions in susceptible individuals. Researchers may also begin targeting TMEM106B in attempts to create treatments that decelerate brain aging.

 Scientists Discover Gene That Determines How Fast Your Brain Will Age
Scientists Discover Gene That Determines How Fast Your Brain Will Age


 Final Messages of the Dying
Final Messages of the Dying
 Will I Die in Pain?
Will I Die in Pain?















