 Researchers at Ben-Gurion University of the Negev in Beer-Sheva, Israel, have developed a promising new treatment for amyotrophic lateral sclerosis, or ALS. Also known as Lou Gehrig’s disease, ALS is a degenerative disease of the nervous system that causes loss of motor function, gradual paralysis, respiratory failure and death. At this time, there is no cure for ALS, and most patients die within two to five years.
Researchers at Ben-Gurion University of the Negev in Beer-Sheva, Israel, have developed a promising new treatment for amyotrophic lateral sclerosis, or ALS. Also known as Lou Gehrig’s disease, ALS is a degenerative disease of the nervous system that causes loss of motor function, gradual paralysis, respiratory failure and death. At this time, there is no cure for ALS, and most patients die within two to five years.
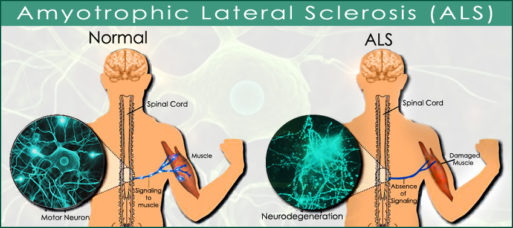
Although the mechanism that triggers ALS is still unknown, researchers have identified a link between increased activity of glial cells and the disease. Glial cells are a type of immune cell that normally supports the function of nerve cells in the central nervous system (the spinal cord and brain). But in people with ALS, they start to damage and eventually kill the motor neurons in central nervous system. They also interfere with their ability to “cleanse” the CNS environment.
The BGU researchers focused on ways to reduce this immune response. Led by Dr. Rachel Lichtenstein of the Avram and Stella Goldstein-Goren Department of Biotechnology Engineering at BGU, they “redesigned” the drug rituximab (MabThera), an FDA-approved treatment for some autoimmune diseases and cancers. In mouse models, the newly created molecule appears to effectively prevent glial cells from attacking the brain.
“Our experimental results on ALS transgenic mice showed a significant increase in life expectancy,” Dr. Lichtenstein said.
Credit: doctortipster.com
Clinical Testing Soon?
Since the FDA has already approved rituximab for use in humans, Dr. Lichtenstein believes there will be few obstacles to its approval for ALS. “We believe that we will only need limited preclinical testing to reach the clinical phase earlier than other initiatives,” she said.
The new drug could also extend the lives of people living with other neurodegenerative diseases, including Alzheimer’s and Parkinson’s disease, said Dr. Ora Horovitz, senior vice president of business development at BGN Technologies. BGN Technologies partners with BGU in efforts to bring new research to the marketplace.
The researchers are currently looking for a pharmaceutical company to assist in developing and marketing the new drug.
Limited Options
Presently, there are only two medicines available for people with ALS who live in the United States. The drug riluzole (Rilutek), which the FDA approved in 1995, works by decreasing the amount of the amino acid glutamate in the brain. However, it typically only slows disease progression by about three to five months.
Credit: drugdevelopmenttechnology.com
The second medication, edaravone (Radicava) just received FDA approval in May 2017. A free-radical scavenger that has undergone clinical trials in Japan since 2015, Radicava is an intravenous infusion that’s given once or twice a week in two-week cycles for a total of 48 weeks. According to data from Japan and MT Pharma America, which markets the drug, patients have achieved statistically significant improvement in function and life expectancy while taking Radicava. However, there is limited data on its long-term efficacy.
To learn more about developments in ALS research, read this report from the 27th Annual International Symposium on ALS/MND (motor neuron disease).

 New ALS Therapy Shows Promise
New ALS Therapy Shows Promise


 Final Messages of the Dying
Final Messages of the Dying
 Will I Die in Pain?
Will I Die in Pain?















